 ADD LIFE –
ADD LIFE –
In the QUEST study, dogs
treated with VETMEDIN lived longer than those on an ACE inhibitor1
The goal of the QUEST1* study was to compare the effects of VETMEDIN and benazepril on quality of life and survival† in dogs with congestive heart failure (CHF) resulting from naturally occurring myxomatous mitral valve disease (MMVD). Recently, investigators analyzed secondary endpoints more closely. The results showed that dogs treated with VETMEDIN lived nearly twice as long1 and required less therapy to maintain quality of life2 as those on an angiotensin-converting enzyme (ACE) inhibitor.
Important safety information
The most common side effects reported in field studies were poor appetite, lethargy, diarrhea, dyspnea, azotemia, weakness, and ataxia. If side effects should occur, pet owners should contact their veterinarian.

Hear an investigator discuss the unprecedented scientific rigor of the
QUEST study.
QUEST was also designed to be completely independent in design and results reporting. Investigators were guaranteed the right to publish study results regardless of the outcome.
VETMEDIN lengthened life
VETMEDIN significantly improved survival time in the QUEST trial.1
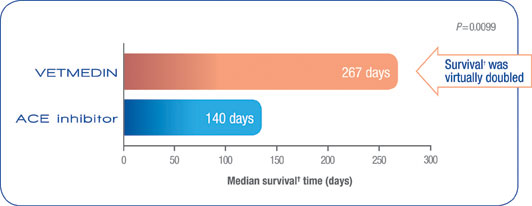
- Dogs treated with VETMEDIN survive† virtually twice as long as the dogs treated with an ACE inhibitor.
- VETMEDIN was well tolerated with few adverse events.
Dogs treated with VETMEDIN required less therapy to maintain quality of life
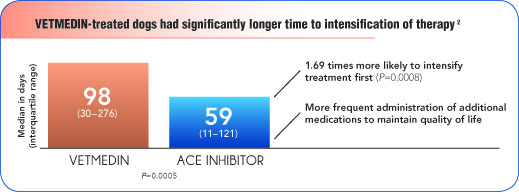
VETMEDIN offers a variety of clinical advantages
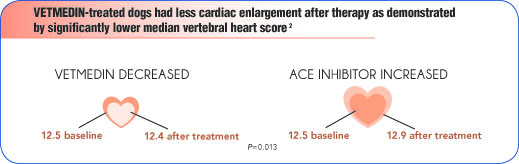
- VETMEDIN also improved body temperature, serum sodium concentration, total protein concentration, and packed cell volume, while reducing fluid retention.2

“This study offers the most compelling evidence to date demonstrating the beneficial effect of pimobendan when compared with benazepril for extending survival in dogs with CHF caused by MMVD when used in conjunction with other standard therapy.”
— Häggström et al, 20081
QUEST study design
QUEST was the largest independent global efficacy trial in canine cardiology history. This trial included 260 client-owned dogs representing 31 breeds. The dogs were recruited at 28 different veterinary centers in 11 countries on 3 continents. Thirty-two leading cardiologists served as investigators.
Hear an investigator’s perspective on the scope of the QUEST trial.
Study parameters were designed to yield robust data. To accomplish this scientific rigor, QUEST was set up as a 3-year, randomized, single-blind, positive-controlled, multicenter, international clinical trial.
Both treatments appeared to be well tolerated with few adverse events
Observed adverse events in both the VETMEDIN (n=18) and ACE inhibitor (n=17) groups were very similar. Gastrointestinal disorders were most common (n=6 and 4, respectively), followed by abnormal behavior, such as lethargy, confusion, or uneasiness (n=3 and 4, respectively).
* Clinical studies were completed using VETMEDIN Capsules. In the US, only the chewable tablets are licensed. Both the capsules and chewable tablets contain the same pharmaceutical ingredient, pimobendan, and are considered equivalent for clinical use. Bioequivalence, however, has not been shown.
† Survival was defined as the composite endpoint of cardiac death, euthanasia due to heart failure, or treatment failure. All dogs received furosemide therapy.
Important safety information
VETMEDIN should not be given in case of hypertrophic cardiomyopathy, aortic stenosis, or any other clinical condition where an augmentation of cardiac output is inappropriate for functional or anatomical reasons.
The safety of VETMEDIN has not been established in dogs with:
- Asymptomatic heart disease
- Heart failure caused by etiologies other than atrioventricular valvular insufficiency or dilated cardiomyopathy
- Dogs younger than 6 months of age
- Dogs with congenital heart defects
- Dogs with diabetes mellitus or other serious metabolic diseases
- Dogs used for breeding or pregnant or lactating bitches
The most common side effects reported in field studies were poor appetite, lethargy, diarrhea, dyspnea, azotemia, weakness, and ataxia. If side effects should occur, pet owners should contact their veterinarian.
References:
1. Häggström J, Boswood A, O’Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22(5):1124–1135. 2. Häggström J, Boswood A, O’Grady M, et al. Longitudinal analysis of quality of life, clinical, radiographic, echocardiographic, and laboratory variables in dogs with myxomatous mitral valve disease receiving pimobendan or benazepril: the QUEST study. J Vet Intern Med. 2013;27(6):1441–1451.

 VETMEDIN is a registered trademark of Boehringer Ingelheim Vetmedica GmbH,
licensed to Boehringer Ingelheim Vetmedica, Inc.
VETMEDIN is a registered trademark of Boehringer Ingelheim Vetmedica GmbH,
licensed to Boehringer Ingelheim Vetmedica, Inc.